Rethinking the current approach to treatment of viral respiratory infections
Historically, pharmaceutical companies have designed drugs to specifically target a single virus. But this one-bug-one-drug antiviral approach is insufficient to address the challenge of treating illnesses caused by the body’s innate immune response to a broad range of difficult-to-diagnose and rapidly changing viruses.
We’re taking a game-changing approach in developing new treatments for COVID-19 and respiratory virus infections
Targeting:
- Activity against COVID-19 and a broad range of respiratory viruses
- Suppressing pro-inflammatory cytokines responsible for symptoms of respiratory virus infections
- Without drug resistance issues that typically plague traditional direct-acting antivirals




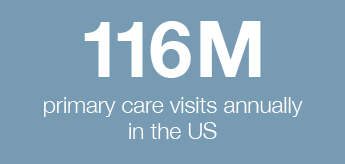

Burden of illness is more pronounced with chronic comorbidities or clinical risk factors
• Asthma, COPD, elderly, young children, immunocompromised